gi
Table of Contents
Esophagus
Lobulated mass in vallecula
- squamous cell carcinoma until proven otherwise
- causes of tracheal penetration include radiation, neuromuscular d/o (CVA, ALS, MS), and prior surgery
- causes of aspiration include diverticulum with stasis, tumor, paresis, and esophageal reflux or obstruction
Esophagitis
- Barrett’s - this is columnar metaplasia of the esophageal epithelium
- occurs in 10% of pts with reflux
- major risk factor for esophageal adenocarcinoma
- pts with hiatal hernia or scleroderma are predisposed
- nodularity (only in distal esophagus), ulceration, focal stricture and a reticular mucosal pattern
- stricture can occur anywhere, but a proximal or mid stricture is especially suggestive
- candida - immunocomp/AIDS or pts with stasis from achalasia or scleroderma
- mucosal nodules in longitudinal columns, and later, diffuse nodularity and plaques giving the “shaggy” esophagus appearance
- DDx includes herpes esophagitis, glycogenic acanthosis, acanthosis nigricans
- superficial spreading squamous ca
DDx Giant ulcer
- most likely causative agents are CMV and HIV
- ulcers occur on a normal background esophagus
- without a history of immunosuppression, should also consider medication induced ulcer
DDx varicoid filling defect
- varices
- uphill are from portal HTN with increased flow via left gastric (coronary) vein into distal esophageal plexus
- downhill varices are from SVC obstruction with collaterals thru azygous
- varicoid carcinoma
- varcies change with change in pt position and amount of esophageal distension, cancer is fixed
- lymphoma
Large intraluminal esophageal tumor
- DDx: spindle cell carcinoma, squamous cell carcinoma, leiomyosarcoma, and fibrovascular polyp
Long smooth strx mid to distal esophagus
- cannot differentiate malignant from benign, and the pt will need endoscopy
- DDx:
- infection - TB and candida
- inflammation - reflux, barrett’s, caustic ingestion, NG tube, radiation (>4500 rads), Crohn’s and epidermolysis bullosa
- neoplasms - mets, lymphoma and primary squamous cell
Inflammatory esophagogastric polyp/fold
- elongated filling defect at GE jxn
- if smooth & club shaped, you don’t need endoscopy, but if lobulated, cannot differentiate from adenomatous polyp or adenoca
Paraesophageal hernia
- GE jxn is below the diaphragm, but part of the gastric fundus extends above the diaphragm thru the esophageal hiatus or a diaphragmatic defect, to the left of the esophagus
- CXR - posterior mediastinal mass, which cannot be distinguished from a hiatal hernia
- UGI - portion of the fundus above the diaphragm, next to the esophagus
Stomach
DDx gastric fold thickening
- gastritis (hypertrophic, alcoholic, infectious (H pylori), and eosinophilic)
- Menetrier’s
- Zollinger-Ellison
- sarcoid
- amyloid
- Crohn’s
- lymphoma (usually more irregular)
- mets and adenoca possible (but less likely)
Gastric ulcer
- location - benign favors the antrum, 75% along lesser curve
- folds - all the way up to the edge of a benign ulcer, but are truncated in a malignant one
- also, folds are normal or uniformly swollen in benign, but are amputated, fused or clubbed in malignant
- benign ulcers project beyond the expected confines of the gastric wall, malignant do not
- benign ulcer is centrally located on its mound, malignant are eccentric
- shape - benign are round or linear, malignant are irregular
- collar - well defined in benign, shaggy/irregular in malignant
- benign have an associated duodenal ulcer in 50%
- carmen meniscus sign signifies a malignant ulcer along the lesser curve, seen on a single contrast compression view
- has a saddle shape
- benign are more often multiple than malignant ones, but still usually solitary
Gastric erosions/apthous ulcers (PIC)
- shallow defects in the mucosal layer of the stomach that do not penetrate beyond the muscularis mucosa
- barium collects in the shallow defect, and there is usually a halo of edema
- peptic - NSAIDS, alcohol, severe burns, steroids, stress
- infectious - CMV, herpes and candida
- Crohn’s - apthoid ulcers
Linitis plastica
- tubular rigid stomach
- usually related to a scirrhous gastric adenocarcinoma
- may also be seen in mets (especially breast cancer)
- lymphoma
- TB
- Crohn’s (ram’s horn)
- sarcoid
- corrosive ingestion
- radiation
DDx of solitary gastric masses
- <2cm
- leiomyoma
- lipoma
- neurofibroma
- hemangioma
- hyperplastic polyp
- ectopic pancreas
- adenomas
- metastasis
- eosinophilic granuloma
- carcinoid
- >2cm
- adenoma
- leiomyoma/leiomyosarcoma
- metastasis
- duplication cyst
- gastric carcinoma
GIST
- benign=leiomyoma and malignant=leiomyosarcoma
- arise from smooth muscle of the gastric wall and grow as submucosal, subserosal or exophytic tumors
- often reach a large size before presenting due to ulceration and GI bleed
- difficult both radiologically and pathologically to distinguish leoimyoma from leiomyosarcoma
- malignant tend to be very large (>10cm) and very heterogeneous with areas of hemorrhage and necrosis
- DDx: adenoca, mets and lymphoma
Gastric perforation
- gastric wall thickening and free air or free fluid should suggest a perforated gastric ulcer
DDx gastrocolic fistula
- ulcers (most common cause)
- gastric and trasnverse colon/splenic flexure cancers
- lymphoma
- Crohn’s
- TB
Gastric Bypass
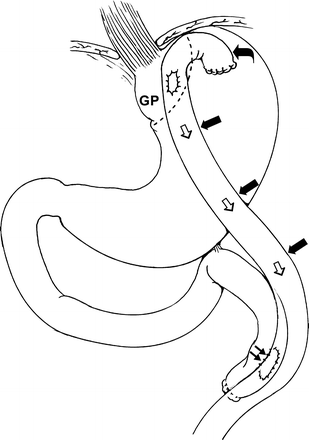
- Diagram of gastric bypass anatomy shows a small gastric pouch (GP) created with stapling (dotted line) of the stomach; anastomosis of the pouch to a Roux-en-Y limb (straight thick black arrows); route of gastric contents to the efferent loop (white arrows); oversewn jejunal loop (curved arrow), or blind loop, of the Roux-en-Y limb; and jejunojejunal anastomosis (thin black arrows).
Small Bowel
Lobulated mass mildly expanding 2nd part of duodenum
- DDx: adenomas (have malignant potential), prolapsing gastric mucosa (bulb only), brunner’s gland adenoma, ectopic pancreatic rest (often with central dimple as in stomach), gastric polyp extending into bulb, leiomyoma, met, lymphoma, adenoca, and flexural pseudotumor
- the more distal a lesion is in the duodenum, the more likely it is to be an adenoma
Duodenal hematoma
- UGI - sharply defined tumor like intramural mass, that narrows or obstructs (usually the 3rd portion) duodenum
Crohn’s disease
- TI fistula - strongly suggestive of Crohn’s, but can also be seen in TB, malignancy and post surgery or radiation
- string sign on SBFT or CT
- skip lesions on a SBFT
- psoas abscess
- DDx TI involvement:
- Crohn’s
- infection (TB, Yersinia)
- neoplasm (lymphoma, mets, carcinoid)
- extrinsic causes such as abscess from appendicitis or tumor
DDx kinking and angulation of SB loops
- TB
- carcinoid
- mets
- radiation - acutely causes fold and wall thickening or fold effacement, chronically causes rigidity, fibrosis, tethering
DDx regular fold thickening in jejunum (HERE)
- defined as >3mm
- intramural hemorrhage
- anticoagulation
- hemophilia
- Henoch Schonlein Purpura
- ITP
- trauma
- vasculitis
- edema - hypoproteinemia, CHF, ischemia, or lymphangiectasia
- radiation
- eosinophilic enteritis (can be regular or irregular)
- abetalipoproteinemia (also could be regular or nodular)
DDx diffuse nodular SB fold thickening (WE CLAIM)
- Whipple’s (remember low density lymph nodes, narrows the DDx to whipple’s vs MAI/TB)
- Eosinophilic enteritis
- Crohn’s dz
- Lymphoma
- Amyloid
- Infections
- giardia and strongyloides (duod and prox jejunum)
- salmonella, yersinia, TB, campylobacter (distal bowel/ileum)
- infections in AIDS include CMV, MAC, cryptosporidium
- Mastocytosis
Celiac Disease
- sensitivity to gluten
- results in malabsorption, steattorhea and diarrhea
- Findings on SBFT
- small bowel dilation
- increased secretions with dilution or flocculation of barium
- decreased number of folds per inch in the jejunum (<3)
- increased number of folds per inch in the ileum (>5)
- fold thickening
- may see transient small bowel intussusceptions
- Moulage sign is caused by dilated loop with effaced folds looking like tube into which wax has been poured
- CT may show adenopathy
- Increased risk of lymphoma and adenoca
Scleroderma involving small bowel
- collagen deposition in the bowel wall
- “Hidebound bowel” which is dilated bowel, but despite being dilated, the folds are close together, with more than 5 folds per inch
- sacculations on the anitmesenteric border (DDx Crohn’s) (true small bowel diverticlui which are on the mesenteric side)
- dilated, atonic duodenum proximal to the aorticomesenteric angle
- pseudoobstruction
- pneumatosis
DDx mesenteric edema and SB thickening, hypodense mesenteric nodes
- mycobacterial infection
- TB - distal small bowel and ascending colon involvement, and usually produces stricturing, fistulas, angulation, kinking, linear and other ulcers
- MAI - usually causes regular fold thickening similar to Whipple’s
- Both TB and MAI cause low density mesenteric or retroperitoneal nodes on CT
- Kaposi’s sarcoma can also produce adenopathy, which may be hypodense
- KS more often causes focal polypoid type lesions with central ulceration (bull’s eye), it can also present as a superficial spreading lesion appearing as thickened folds
- metastatic disease from a mucinous adenocarcinoma or seminoma
- lymphoma can cause the findings of wall thickening, edema and adenopathy, although the nodes are not typically hypdense, but may rarely be so when treated
- Whipple’s - nodular fold thickening in the duodenum and proximal jejunum
SMA thrombosis
- bowel wall thickening, which may be homogeneous or targetoid with separate visualization of the layers of the bowel wall
- intramural gas/pneumatosis is seen in 30%, but is not specific for infarction
- gas is seen in mesenteric veins and portal veins in about 13%
- infarcted segment acts like an obstruction, and the bowel proximal to it is dilated and filled with gas and fluid
- can be some ascites
Meckel’s diverticulum
- persistence of the omphalomesenteric duct
- antimesenteric border, within 100cm of the IC valve
- less than half have ectopic gastric mucosa, and these can be functional and cause bleeding
- complications - obstruction from intussusception, diverticulitis (may mimic appendicitis), enterolith, perforation and neoplastic transformation
Cecum and TI narrowing
- Crohn’s
- TB
- adjacent inflammatory processes such as appendicitis and diverticulitis
- neoplasms such as adenoca, mets and lymphoma
Colon
Toxic Megacolon
- marked dilation and thickening of the transverse colon, with a nodular haustral pattern
- can resemble pseuodpolyps
- transverse colon is the most dilated (most nondependent area)
- usually the result of fulminant IBD
- can also be related to ischemia
- infectious colitis(esp in AIDS)
- pseudomembranous colitis
- perforation is pretty common and is the most dreaded complication
- look for subtle free air on the supine XR of the abdomen
Lymphoid hyperplasia
- small nodules, approxiately 3mm in diameter, and are mostly found in the ileocecal region and rectum
- often have a small central dimple filled with barium
- in children, normal finding
- in adults, it may be related to an adjacent infectious or inflammatory process and has been seen in pts with infections such as giardiasis, IBD, and hypogammaglobulinemia
Ischemic colitis of IMA territory
- descending and distal TRV
- most common area to experience ischemia, especially around the splenic flexure (watershed area)
- more often related to hypoperfusion from a low flow state than it is to thrombus/embolus
- most episodes occur in the elderly, and resolve without sequella
- infection and perforation can result
- mucosa and submucosa are most suceptible
- Findings:
- marked segmental wall thickening resulting in thumbprinting
- ulceration of the mucosal surface
- pneumatosis intestinalis may also be seen, and portal venous gas in severe cases
- healed ischemia can produce a stricture or fibrosis with loss of haustra
Cecal volvulus
- 2nd most common type of colonic volvulus; sigmoid is most common
- can only occur if the right side of the colon is incompletely fused to the retroiperitoneum(hypermobile cecum)
- KUB - single large dilated loop (which by its size must be colon), which usually lies upward and to the left
- rest of the colon is not dilated
- small bowel usually is dilated
- BE shows a bird’s beak at the site of obstruction
- 2 types of cecal volvulus, axial torsion and bascule
- axial is the twisting type
- bascule means the cecum folds on the right colon without twisting
DDx ascending colon wall thickening
- typhlitis - most commonly involves cecum and ascending coon, but can involve TI
- usually the result of hematologic malignancy
- colon cancer - can have multiple appearances, one of them is wall thickening
- lymphoma - what can’t it look like?
- metastasis
- ischemia - not the most common pattern to just involve ascending colon, but it could
- Crohn’s - would be odd not to have any other findings
- infection such as ambiasis, which favors the proximal colon, TB, actinomycosis
- appendicitis can cause ascending colon wall thickening d/t proximity
Apthoid ulcers
- in the colon or small bowel, apthous ulcers can be due to Crohn’s (most common), or infections, such as viral, Amebiasis, salmonellosis or ischemia and less likely Behcet’s
- Behcet’s → triple-symptom complex of recurrent oral aphthous ulcers, genital ulcers, and uveitis
- bull’s eye appearance like apthous ulcers elsewhere in the GI tract
- small bowel stricture that makes Crohn’s even more likely, although some infections could produce that too
Appendicitis
- KUB - appendicolith is seen in about 10%
- other findings include SBO or ileus, obscuration of psoas or obturator margins, abscess seen as mass with displacement of bowel loops, +/-mottled gas in it
- BE - if the entire appendix fills, that rules out appendicitis
- nonfilling of the appendix occurs in about 20% of nl BEs
- visualization of an abscess filling with contrast next to the appendix is rare, but helpful
- nonfilling of the appendix and a mass/mass effect in the lower abdomen/pelvis
- CT - dilated/thickened appendix, with diameter > 6mm, increased appendiceal wall enhancement, stranding in the periaapendiceal fat, appendicolith, and abscess or phlegmon
Cobblestoning in colon
- typical for Crohn’s
- pseudopolypoid islands of thickened mucosa and submucosa in between areas of deep linear ulcers and transverse fissures
- UC can produce a somewhat similar appearance (but not called cobblestone) due to pseudopolyps on a background of ulceration
- if involves the entire colon, I would probably favor UC over Crohn’s, though Crohn’s could involve the entire colon too
- DDx: pancolitis from ischemia or infection
Villous adenoma
- tubular, tubulovillous or villous, with villous having the highest malignant potential
- villous ones are almost always sessile and more lobulated than tubular adenomas
- frondlike or carpet lesions are almost always villous
- 50% of all adenomas occur in the rectosigmoid area
- majority of colon cancers arise from adenomatous polyps
- risks being: <5mm=<0.5%, 5-10 mm=1%, 10-20mm=10% risk, and >2cm has 50% risk
- radiographic feautres suggestive of malignancy include an irregular, lobulated surface, broad base>height, retraction of colon wall, and interval growth
- classic radiologic findings of polyps
- bowler’s hat results when a polyp forms acute angles with the bowel wall, and therefore traps barium around it’s base, forming a white ring, and the barum on the surface (dome) of the polyp forms a curvilinear denisty
- mexican hat sign is when you see a polyp with a stalk end on through the head of the polyp, so that the stalk forms a white ring inside the other white ring which is barium on the head of the polyp
Sessile colonic polyp
- sessile polyp = a polyp without a stalk, vs pedunculated which has a stalk
- sessile have a higher risk of malignancy than pedunculated
Colon CA staging
- A=limited to mucosa
- B1=extension into but not thru muscularis propria
- B2=thru muscularis propria, into serosa or mesenteric fat, no nodes
- C1=into, not thru, muscularis propria with nodes
- C2=thru muscularis propria with nodes
- D=distant mets
Rectal CA staging
- Tumor categories
- Tx:No description of the tumor's extent is possible because of incomplete information.
- Tis: In situ carcinoma; the tumor involves only the muscularis mucosa
- T1: The cancer has grown through the muscularis mucosa and extends into the submucosa
- T2:The cancer has grown through the submucosa and extends into the muscularis propria
- T3: The cancer has grown through the muscularis propria and into the outermost layers of the colon but not through them; it has not reached any nearby organs or tissues
- T4a: The cancer has grown through the serosa (visceral peritoneum)
- T4b: The cancer has grown through the wall of the colon and is attached to or invades nearby tissues or organs
- Node categories
- Nx:No description of lymph node involvement is possible because of incomplete information
- N0:No cancer in nearby lymph nodes
- N1a:Cancer cells found in 1 nearby lymph node
- N1b:Cancer cells found in 2 to 3 nearby lymph nodes
- N1c:Small deposits of cancer cells found in areas of fat near lymph nodes, but not in the lymph nodes themselves.
- N2a:Cancer cells found in 4 to 6 nearby lymph nodes
- N2b: Cancer cells found in 7 or more nearby lymph nodes
- Metastasis categories
- M0:No distant spread seen
- M1a: The cancer has spread to 1 distant organ or set of distant lymph nodes
- M1b:The cancer has spread to more than 1 distant organ or set of distant lymph nodes, or has spread to distant parts of the peritoneum
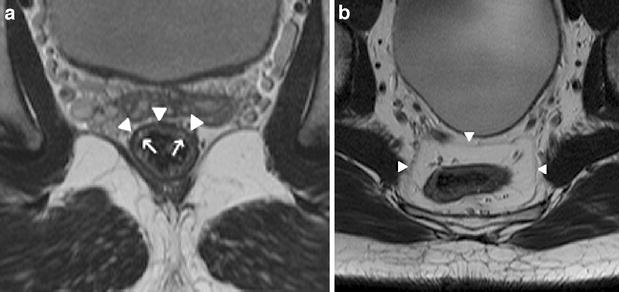
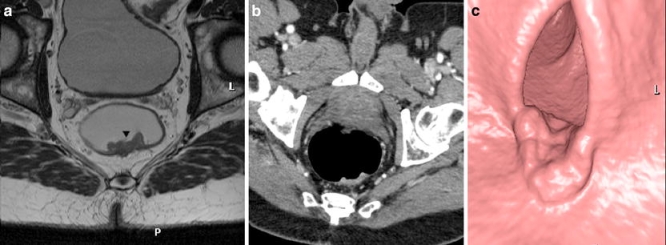
- a) Axial T2-weighted FSE (TSE) sequence of the pelvis depicting the layers of the rectal wall. The mucosa and submucosa can be visualized as a relatively hyperintense band (arrows). The hypointense line (arrowheads) represents the muscularis propria.
- b) Axial T2-weighted FSE (TSE) sequence. The mesorectal fascia can be visualized as a thin line (arrowheads), enveloping the mesorectal compartment, containing the rectum, mesorectal fat, blood vessels, lymphatic vessels and nodes
- a Paraxial T2-weighted FSE (TSE) sequence. T1/2 rectal cancer. The relatively hyperintense intraluminal tumor (arrowhead) is confined to the rectal wall. Tumor invasion of the mesorectum is not visible.
- b Paraxial 3D-MPR and
- c intraluminal (virtual endoscopy) CT reconstuctions after rectal insufflation of CO2 showing the same tumor as a
Filiform polyps
- filiform polyps are also called postinflammatory polyps
- result of healing aorund a pseudopolyp, which leaves a projection of normal mucosa, which is usually comma shaped
- can be seen on a background of normal mucosa or of recurrent disease.
- most often from UC, but can also be seen in Crohn’s or other inflammatory conditions of the colon
C Dif (pseudomembranous) colitis
- should not do a BE in a pt if you suspect C. dif clinically or b/c of KUB findings of marked bowel wall thickening, nodularity, and thumbprinting (risk of perforation)
- BE - shaggy, irregular colonic contour due to pseudomemebranes with barium filling the clefts b/w them
- DDx - ischemia and UC
- CT - classic appearance is the accordion sign, which is intraluminal contrast interposed b/w markedly thickened haustra
- marked bowel wall thickening (averages 15mm) involving the entire colon (which makes ischemia less likely)
- low attenuation in the bowel wall and inflammatory stranding in the surrounding fat
Lead pipe colon
- colon which is short, narrow, tubular, and lacks haustrations
- usually a result of chronic UC, which causes hypertrophy and fixed contraction of the muscularis mucosa
- DDx - cathartic colon
Gardner’s syndrome
- autosomal dominant
- causes multiple adenomatous polyps (very high risk of colon cancer → 100%)
- all have polyps in colon, and 90% have some in duodenum, with 10% having them in the stomach
- extra-intestinal findings of sinus osteomas, epidermoid cysts and abdominal desmoids
- desmoids are soft tissue density masses which often have spiculated margins
- locally aggressive and often recur after removal, but do not metastasize (not malignant)
- treatment is prophylactic colectomy at around age 20 to prevent colon cancer
Virtual Colonoscopy
Technique
Insufflation
• CO2 improves distention and decreases discomfort (rapid reabsorption) • Automated improves distention • Start @ 15mm Hg & gradually increase to 20-25mm Hg • Higher (25mm Hg) needed for larger pts
Tagging
• Fecal - barium • 3 oz morning • 3 oz noon • 3 oz evening • Fluid - iodine • ? dose
Positioning
• R lat decub • Prone (optional) • L lat decub (optional) • Supine • Scan 2 positions (usually supine & prone) • If failed colonoscopy, do lat decubitus view • Finish by deflating balloon, removing catheter, and scanning lower 10cm
Scanning
• Detector collomation: 0.6-0.75mm • Slice thickness: 1.0-1.25mm • Recon interval: 0.8-1.0mm • Total dose: 5-6.5 mSv
Interpretation
• Windows - 2000/0 • 2D - Use all 3 recon views • Use 3D for troubleshooting • Tagging often sticks to outside of polyps
Cancer risk
• <= 5mm - 0.08% • 6-9mm - 0.7% (consider 3 yr f/u) • >= 10mm - 15.7%
Liver
Cirrhosis
- earliest phases of liver injury - the liver becomes fatty and enlarged
- later stages - the liver is small and nodular
- regenerating nodules may be present, and these are isodense precontrast and hypodense post contrast
- contrast enhancement is inhomogeneous and diminished vs nl
- porta hepatis and intrahepatic fissures are more prominent than usual b/c of hepatic atrophy
- hepatic and portal veins are often difficult to see b/c they are compressed
- KEY findings - increase in the caudate to right lobe ratio
- ratio of >.65 is 96% confidence of cirrhosis (nl is about .37)
- right lobe atrophies and the caudate and lateral left lobe enlarge
- chiladiti syndrome where colon interposes b/w liver and anterolateral abd wall or diaphragm is much more common in cirrhosis
- CT - may show arterial portal shunting, with early filling of one portal vein, and increased enhancement of the lobe it supplies
- often increased density in the mesenteric fat from edema when compared to the retroperitoneal fat
- findings of portal HTN include ascites, splenomegaly, and varices
- varices visible on CT include paraesophageal, varices in gastrohepatic ligament and retroperitoneum, recanalized periumbilical vein, gastrorenal and splenorenal veins
- look for HCC!
Hepatocellular carcinoma
- 80% of HCCs arise in cirrhotic livers
- major risk factors include alcoholic cirrhosis, hepatitis, parasitic infection, & hemochromatosis
- most pts are >50
- high AFP level
- tumor can be focal(most common), multifocal or infiltrative
- most are hypodense on NCCT and enhance prominently in the arterial phase, b/c HCCs have more hepatic arterial flow than nl liver, which derives 80% of its flow from the portal system
- necrosis is common, and 25% have Ca+
- frequently invades the portal and/or hepatic veins and IVC
HCC Features
- Masslike configuration
- Arterial hyperenhancement
- Portal venous phase or later phase hypoenhancement
- Increase size by 10 mm or more in 1 year
- Tumor within vein lumen
Focal Nodular Hyperplasia
- second most common benign tumor of the liver (after hemangioma)
- occurs mostly in women, but is NOT associated with birth control pills
- only liver tumor with Kupffer cells
- Tc SC scans 60% are normal uptake or hot(very helpful), 40% are cold(not helpful)
- almost always have uptake on HIDA scans
- often have a central scar, which does limit the DDx to FNH vs HCC, especially fibrolamellar type
- NCCT - hypodense and homogeneous except for the scar
- CECT - intense enhancement during the aretrial and portal phases, with rapid washout except in the scar, which retains contrast so that on delayed imaging the scar is hyperdense
- Angio - hypervascular and have a spokewheel appearance
- MRI, FNHs have distinctive features
- often iso or only slightly hyperintense on T2(as opposed to malignant tumors) but the scar is hyper on T2
- GRE, the vessels in the central scar are bright
- Gad - have rapid filling and rapid washout, and the scar accumulates contrast, especially on delayed imaging
- scar in an HCC is dark post contrast b/c it is necrotic tissue
Hepatic adenoma
- hepatic adenomas tend to occur in young females on birth control, with anabolic steroid use, or Type 1 GSD
- large, >10cm, and solitary
- usually bleed internally, but can rupture into the peritoneum
- CT - usually hypodense (often very hypodense, almost like fat), but can have hyperdense areas related to hemorrhage
- CECT - peripheral and then centripetal enhancement similar to hemangioma, except that there is rapid washout, as opposed to hemangioma which retains the contrast
- MR - can be unique on T1 b/c they are usually bright due to the presence of fat, and may have additional bright areas related to hemorrhage
- often heterogeneous due to hemorrhage and necrosis
- dilated vessels at the periphery
- US - more often hyperechoic, and can have central hypoechoic areas of necrosis and hemorrhage
- enlarged peripheral vessels can sometimes also be seen on US
Liver hemangioma
- second most common solid mass in the liver, exceeded only by mets
- affect all ages, women>men
- 10% are multiple
- most are asymptomatic, but giant hemangiomas may cause symptoms fom mass effect, hemorrhage or arteriovenous shunting
- CT - well defined hypodense mass with density like blood veesels, peripheral globular enhancement with gradual filling in, retention of contrast on delayed imaging
- may have areas of necrosis or hemorrhage which will not enhance
Hepatic abscess
- hepatic abscesses may be bacterial, parasitic or mycotic
- pyogenic ones are gram negative and come from the biliary tree
- other routes are hematogenous from portal vein or hepatic artery, and direct spread from adjacent organ or trauma
- 80% are in right lobe
- 2/3 of cases are multiple, and smaller abscesses are more likely to be multiple
- CT usually shows a hypoattenuating lesion with an enhancing rim
- 20% have gas inside
- many have septations
- amebic abscesses are usually peripheral in location, in the right lobe, and tend to be unilocular
- echinococcal (hydatid) abscesses usually have smaller daughter cysts within a larger cyst
- detached cyst membrane may float in the cyts, producing the “water lily” sign, which is highly specific for echinococcus
Focal fat in caudate
- fatty liver is a common response of hepatocytes to injury or toxins and has many causes, including diabetes, pancreatitis, obesity, alcoholism, malnutrition, steroids, glycogen storage dz, and chemotherapy
- most common regions involved are the medial segment of the left lobe of the liver (adjacent to lig teres) and adjacent to the GB
- CT - hypodense
- in and out of phase GRE - loss of signal on the out of phase
- Features:
- geographic borders, interdigitation of the borders with the adjacent nl liver, lack of mass effect, especially on the hepatic veesels, and rapid change over time (can develop and resolve in weeks)
DDx multiple hyperechoic liver lesions on US
- mets, usually from a GI primary, especially colon ca
- multifocal HCC
- multiple hemangiomas
- multiple adenomas, lipomas or FNHs
DDx Multiple calcified liver lesions
- infections, especially prior granulomatous like histo, but also TB, ricketsia and others, parasites, and PCP
- multiple abscesses and echinococcus can Ca+
- metastatic dz from mucinous tumors of the colon, breast, ovary, stomach, melanoma or osteosarcoma
- multiple Ca+ HCCs or hemangiomas, cysts or multiple Ca+ aneurysms
DDx Multiple low density / high T2 liver lesions
- hemangiomas
- complex cysts
- mucinous metastases (colon, ovary) or cystic metastases (melanoma, breast, lung)
DDx Diffusely abnormal liver enhancement
- Budd-Chiari syndrome
- Congestive hepatopathy
- Nutmeg pattern of enhancement
- Venous enlargement
- Uniform enhancement on delayed phase
- Diffuse HCC
- Large lesion
- Permeative in appearance
- Heterogeneous arterial enhancement
- Variable washout on delayed phase imaging
- Primary sclerosing cholangitis
- Intrahepatic biliary dilation
- Liver atrophy, caudate hypertrophy
- Increased peripheral enhancement
Portal vein thrombosis
Causes
- idiopathic
- cirrhosis and portal HTN
- tumors such as HCC, liver mets or panc ca
- if you see thrombus in a portal vein, hepatic vein, IVC, or atrium, always check for HCC, RCC or adrenal carcinoma in adults, and wilms and hepatoblastoma in kids
- vascular causes such as Budd Chiari
- passive congestion of the liver from CHF, P vera, hypercoagulable state, sepsis, & abdominal abscess
CT signs
- Increased size of PV, diameter >13mm
- Low denisty thrombus in PV
- PV is not enhancing or is not visualized
- calcification in the portal vein in the thrombus or the wall - chronic
- cavernous transformation - chronic
- portal HTN - chronic
Budd Chiari
- obstruction of hepatic venous outflow, either at the level of the hepatic veins, or in the IVC above the diaphragm
- primary - obstructing membrane seen in the far east
- secondary - can be from HCC, hepatic mets, RCC, oral contraceptives, chemo, P vera, hypercoagulable state, ¶sites in liver
- thrombus may be visible in the hepatic veins or IVC on US, CT or MR
- CT - liver is enlarged and has decreased density peripherally relative to the central aspects and caudate lobe
- reversal on delayed images, as contrast washes out centrally but persists peripherally, so that now the periphery is hyperdense (also seen in CHF and constrictive pericarditis)
- hypodensity(lack of flow) and lack of enhancement in hepatic veins in the hepatic venous phase of contrast
- caudate lobe is spared b/c it has separate venous drainage to the IVC, and eventually the caudate enlarges
Multiple hypodense solid hepatic lesions
- mets vs multifocal HCC
- less likely possiblities would include multiple abscesses, hemangiomas or adenomas
- look for evidence of cirrhosis or portal vein invasion which might sway you toward HCC, whereas a primary elsewhere on the scan which would lead you to mets, or gas which would suggest abscesses
- need history for infection or primary cancer
- AFP might also help distinguish mets vs HCC, but ultimately you may need a biopsy
Hemochromatosis
- Primary form involves multiple organs → liver, pancreas, heart, bone marrow
- Secondary form involves reticuloendothelial system organs only (liver and spleen)
Biliary
- Short, smooth strictures are usually benign
High Bile Duct Stricture
- Klatskin tumor
- Gallbladder CA
- HCC
- Metastatic nodes
Mid CBD smooth stricture
- smoothly tapered strictures usually have a benign etiology and abrupt ones have a malignant etiology
- DDx of a biliary stricture must include benign and malignant causes
- Causes:
- inflammatory - pancreratitis, stone passage, ischemia, or recurrent infection/cholangitis
- trauma from surgery or otherwise
- extrinsic compression - pancreatitis, mets(lung, breast and GI) in porta hepatis, peripancreatic or periduodenal, nodes from lymphoma or reactive, Mirizzi’s, GB cancer, liver tumor
- cholangiocarcinoma
Distal Bile Duct Stricture
- Pancreatic CA
- Cholangio CA
- Ampullary CA
- Duodenal CA
Causes of Bile Duct Thickening
- sclerosing cholangitis → associated w/ ulcerative colitis
- bile duct stones
- indwelling stents
- AIDS cholangitis
- oriental cholangiohepatitis
- pyogenic cholangitis
- choloangiocarcinoma → associated with luminal obliteration
- pancreatitis
Cholangitis
- Saccular outpouchings of intrahepatic bile ducts → ascending cholangitis
Sclerosing cholangitis
- progressive inflammatory process that usually involves the biliary system diffusely
- more common in men and is associated with UC in 70% of cases (but only about 10% of UC pts get SC)
- assoicated conditions include Riedel’s thyroiditis, retroperitoneal and mediastinal fibrosis, and Crohn’s
- diffuse or localized areas of narrowing and relative dilation (beaded appearance) involving both the intra and extrahepatic ducts
- may have a “pruned tree” appearance, resulting from dimished arboriztaion of intrahepatic ducts
- cholangioca is a complication
- DDx: AIDS cholangiopathy - similar picture, and cannot be distinguished on radiology alone
Cholecystitis with perforation
- CT findings in acute cholecystitis
- Gallstones in 95%
- Hydropic GB, trv diameter >5cm 3.GB wall >3mm thick
- Halo of subserosal edema in GB wall
- Pericholecystic fluid, which signifies perforation
- Increased bile density >20HU
- Stranding in the pericholecystic fat
- Increased enhancement of GB wall
- Air in lumen or wall if emphysematous
Mirizzi’s syndrome
- stone is impacted in the cystic duct or gallbladder neck, with the mass effect from the stone and inflammation causing compression and narrowing of the adjacent CBD or CHD
- at the level of the cystic duct (Type 1)
- or a stone actually erodes int o the CBD (Type 2)
- narrowing is usually eccentric, worse on the right side, near where the stone is
- intrahepatic ducts may be dilated d/t obstruction
- usually cholecystitis due to the GB/cystic duct stone, and therefore the ERCP shows absent or impaired filling of the GB
- if not recognized, the surgeon may go in and mistake the common hepatic duct for the cystic duct and ligate the CHD
Cholangiocarcinoma
- Sclerosing form of cholangio CA looks identical to PSC and asian cholangiohepatitis
Klatskin tumor
- cholangiocarcinoma arising at the confluence of the right and left hepatic ducts
- can have multiple appearances
- scirrhous pattern - form strictures
- polypoid masses projecting into the lumen
- liver masses, similar in appearance to other liver neoplasms
- extrahepatic cholangiocas tend to have a better prognosis than intrahepatic
- overall prognosis is poor b/c they invade the portal vein and bile ducts
- Klatskin tumor is usually the scirrhous type that grows along ducts and thickens their walls
- CT - mass in the region of the porta hepatis
- lobar atrophy with dilated intrahepatic ducts is very characteristic for a cholangiocarcinoma
- US - duct dilation, isolation of the right and left duct segments (meaning they never communicate), mass or bile duct wall thickening at the hilus, and lobar liver atrophy with crowded dilated bile ducts
- US may not show a mass, only thickening, or just obstruction to the level of the porta hepatis but not distal
Cholangiocarcinoma in sclerosing cholangitis
- cholangiocarcinoma complicates PSC in about 15%, and the already abnormal ducts can mask a carcinoma
- an area with more marked dilation than the other areas, interval stricture formation and biliary dilation on f/up imaging (usually progresses slowly), filling defects >1cm, or dominant stricture suggests cholangioca
- REMEMBER to look for cholangioca in any SC case
Choledochocele
- protrusion of dilated intramural CBD into the duodenum, similar to a ureterocele
- smooth sac like dilation of the intramural segment of the CBD
- barium studies show a smooth well defined intraluminal filling defect in the region of the papilla that changes in shape with compression and peristalsis and does not fill with contrast
Pancreas
DDx Cystic Lesion of the Pancreas
- unilocular
- pancreatic pseudocyst
- intraductal papillary mucinous neoplasms (IPMN)
- serous cystadenoma uncommonly uni/macro locular
- pancreatic cysts occur in association with
- von Hippel Lindau syndrome
- autosomal dominant polycystic kidney disease (ADPKD)
- cystic fibrosis
- macrocystic : multilocular
- mucinous cystic neoplasm of pancreas : usually body and tail
- intraductal papillary mucinous neoplasms (IPMN)
- serous cystadenoma uncommonly uni/macro locular
- acinar cell cystadenocarcinoma
- microcystic
- serous cystadenoma : usually head. 30% have central scar
- cystic with a solid component
- macrocystic tumours can also have a solid component
- solid pseudopapillary tumour of pancreas
- primary ductal pancreatic tumour with cystic degeneration
- cystic degeneration of islet cell tumours
- insulinoma
- glucogonoma
- cystic teratoma
- metastases to pancreas
Distinguishing features of pancreatic cystic lesions:
| Typical characteristics | IPMN | MCN | SC | PSEUDO | SPN | LEC | cNET | cPDAC |
|---|---|---|---|---|---|---|---|---|
| Age group | Elderly | Middle | Middle-elderly | Any | Young | Elderly | Middle-Elderly | Elderly |
| Gender | >50% male | 95% female | >50% female | >50% male | 80%–90% female | 80% male | 50% each | >50% male |
| History | Asx; pain; jaundice | Asx; Pain; nausea | Asx; VHL | Pancreatitis | Asx; pain; nausea | Asx | Asx; Fxnl; MEN | Asx; pain; ± jaundice |
| % of all cysts | 17%–40% | 9%–28% | 7%–36% | 1%–19% | 1%–13% | <2% | <8% | 13%–16% |
| Location in pancreas | Head in 70%; multifocal | Body/Tail in 95% | Anywhere | Anywhere | Anywhere | Peripheral | Anywhere | Anywhere |
| Shape | Ovoid | Spheroid | Ovoid | Spheroid | Ovoid | Ovoid | Spheroid | Variable |
| Locularity | Any | Uni- or oligo- | Oligo- or multi- | Uni- | Oligo- or Multi- | Oligo- | Uni- | Any |
| Duct communication | Common | No | No | Common | No | No | No | Some |
| Calcification | No | No | Central sunburst | No | Some | No | Some | No |
| Cyst fluid appearance | Viscous, clear, muc. | Viscous, clear, muc. | Thin, clear, nonmuc. | Opaque, bloody/ necrotic debris | Opaque, bloody/ necrotic debris | Nonmuc., crystalline debris | Nonmuc. | Thin |
| High CEA/Mucin | + | + | − | − | − | − | − | ± |
| High Ca19-9 | ± | ± | − | − | − | − | − | ± |
| High amylase | + | − | − | + | − | − | − | ± |
| Epithelium | Columnar, papillary | Columnar | Cuboidal | No epithelium | Poorly cohesive cells with nuclear grooves | Squamoid | Uniform | Gland-forming |
| Stroma | Fibrotic | Ovarian | Fibrotic | Fibrotic | Sometimes hyalinized | Lymphoid | Sometimes hyalinized | Fibrotic |
* Abbreviations: IPMN: intraductal papillary mucinous neoplasm; MCN: mucinous cystic neoplasm; SC: serous cystadenoma; PSEUDO: pancreatic pseudocyst; SPN: solid-pseudopapillary neoplasm; LEC: lymphoepithelial cyst; cNET: cystic neuroendocrine tumor; cPDAC: pancreatic ductal adenocarcinoma with cystic degeneration; VHL: von Hippel-Lindau disease; muc.: mucinous; Nonmuc: nonmucinous; Asx: asymptomatic; Fxnl: functional.
Cystic Pancreatic Masses
- Cystic lesion on CT–> get MRI/MRCP to characterize.
- If Simple unilocular cyst < 2 cm (maybe 3 cm 35/36 were benign at this size in one study) then follow up. Studies have shown these cause no morbidity/mortality.
- If micro/macrocystic and < 4 cm then follow. Many of us may not be comfortable calling a serous cystadenoma.
- Otherwise EUS/FNA for diagnosis and then appropriate management can be determined.
- <1cm – repeat CT 1 year
- 1-2cm – repeat CT 6-12 months
- >2cm – repeat CT in 3-6 months
CT findings in acute pancreatitis
- focal or diffuse gland enlargement
- focal or diffuse decrease in gland density
- blurring of pancreatic margins from inflammation
- stranding/inflammatory changes in the peripancreatic fat
- thickening of retroperitoneal fascial planes such as gerota’s or lateral conal fascia
Pancreatitis with pseudocyst vs abscess
- abscess will have a thicker wall than a pseudocyst
- abscess may have gas
- any contained fluid collection could be an abscess, so you need to rely on clinical signs of infection
- most pseudocysts are in and around the pancreas, but they can be almost anywhere, including in the liver, spleen, or thorax
- most are well defined, smooth walled
- often have septations/mulitloculations
- smaller ones resolve on their own, but once 6cm this becomes unlikely
- complications include rupture bleeding and infection
Chronic pancreatitis
- pancreatic fibrosis and atrophy
- likely secondary to alcohol or gallstones
- Ca+ in the distribution of the pancreas
- irregular dilation of the PD with strictures and stenoses, and there may be filling defects from stones
- may also be a CBD stricture, and inflammatory extrinsic impressions on the duodenum
- pancreas may be small, diffusely or focally enlarged, or heterogeneous
- CT can show duct dilation, Ca+ and gland atrophy
- ERCP can show duct strictures & dilations and side branch enlargement
- pseudocysts may be present
DDx multiple pancreatic cysts or masses
- cysts - VHL or ADPCKD (VHL much more likely to involve pancreas than is ADPCKD), parasitic like echinococcus, multiple pseudocysts from pancreatitis, or cystic mets (RCC, lung, breast,melanoma, HCC ovarian)
- solid - mets, multiple primaries (such as multiple islet cell tumors in MEN) or rarely lymphoma
Pancreas Divisum
- congenital anomaly
- failure of fusion of the dorsal and ventral pancreatic buds
- either entirely separate or with a thin channel connecting them
- dorsal duct (Santorini) drains the body, tail and superior portion of head into the minor papilla
- ventral duct (Wirsung) drains the inferior head and uncinate via the major papilla
- at ERCP, if only the major duct is cannulized, only the short ventral duct fills
- to see the dorsal duct, the endoscopist needs to cannulate the minor papilla
- CT may show 2 ducts, or separation of the ventral and dorsal portions of the pancreas by a fat cleft, or lobulation of the pancreatic head
- higher risk of pancreatitis b/c the bulk of the bile is drainng via a small duct
Annular pancreas
- pancreas encircles the second portion of the duodenum due to an abnormality in the rotation of the pancreas or the duodenum
- major complications are pancreatitis and partial obstruction of the duodenum
Pancreatic head mass with biliary dilation
- pancreatic adenocarcinoma vs pancreatitis
- cannot be distinguished on imaging alone
- typical findings for adenoCA:
- focal enlargement of pancreas
- calcifications are RARE in pancreatic adenoCA
- hypoechoic/hypodense/hypointense mass ± duct dilation and atrophy of adjacent parenchyma
- double duct sign=dilation of both PD and CBD, which is suggestive of pancreatic adenoca, but is NOT specific
- UGI may show compression or invasion of stomach, duodenum or transverse colon
- risk factors for adenoca include smoking, alcohol, diabetes, pancreatitis, family history and high fat diet
- nonresectability: vascular encasement of SMA, celiac etc, liver mets, adenopathy, ascites and invasion of adjacent organs
Cystic mass with Ca+ in pancreatic tail
- if the Ca+ is inside the mass, then this would most likely be a serous cystadenoma
- 1/3 of these have amorphous central Ca+ (it is the pancreatic tumor which most often Ca+)
- can occur in any portion of the pancreas, and tends to occur in pts over 60, F>M
- composed of numerous minute cysts separated by septations
- NOT premalignant
- CECT - swiss cheese appearance b/c of the enhancing septae +/- Ca+
- other possibilties - necrotic adenoca or nonfunctioning cystic islet cell tumor, cystic mets from a mucinous source & cystic teratoma(rare)
- if the Ca+ is in the rim or in septations, this could be a pseudocyst, or a mucinous cystadenoma
- compsed of <6 cysts, with each cyst >2cm
- tumor of elderly females
- CT - multiolocular or unilocular, usually in the tail
- may have septae, papillary projections or mural nodules
- malignant or premalignant
- 15% have curvilinear wall Ca+
Serous cystadenoma in pancreas
- US - lobulated solid mass with mixed hypoechoic and echogenic areas, with the cysts being too small to resolve
- central scar may be seen as an echodensity
- NCCT - hypodense and lobulated in contour
- CECT - swiss cheese appearance b/c of tiny cysts
- central Ca+ may be seen and the central stellate scar may be seen too
- asymptomatic pts or pts who are a high surgical risk can avoid surgery b/c it is a benign lesion
- treatment is surgical
Pancreatic necrosis
- devitalized pancreatic tissue secondary to acute pancreatitis and resultant ischemia
- diffuse or localized decrease in density of pancreatic parenchyma, WHICH DOES NOT ENHANCE post contrast
Pancreatic calcifications w/ cirrhosis
- alcoholic or former alcoholic who has chronic panreatitis and cirrhosis, both of which are sequella of alcohol abuse
- bile duct stones, such as in oriental cholagiohepatitis etc, and has biliary cirrhosis and pancreatitis from stones as well
Young pt w/ mass in pancreatic head w/ calcification
- solid and papillary epithelial neoplasm. There are not many pancreatic tumors that occur in a pt this young
- for SPEN, 24 is the mean age
- more common in black women
- peripheral Ca+ may be seen on plain film or CT
- US - well demarcated solid mass with hypoechoic areas (so it is mixed solid/cystic) d/t necrosis and hemorrhage
- CT - muscle density mass with hypodense areas
- may look like a thick walled cyst with a ragged inner margin
- low grade malignancy and is curable with surgery
- DDx: islet cell tumor, which can Ca+ when large, teratoma or hemangioma
DDx dilated panc duct
- chronic pancreatitis
- usually also has areas of bandlike narrowing, such that the PD looks beaded
- side branches are often dilated or blunted
- mass
- duct dilates proximal to a malignant stricture or mass
- often associated with atrophy of the adjacent glandular tissue
- aging, but this only produces mild dilation due to glandular atrophy
- in the setting of a huge PD, I would only mention chronic pancreatitis and malignancy
Miscellaneous
Patterns of Attenuation in Bowel Wall Thickening
Homogeneous
- Common
- Submucosal hemorrhage
- Lymphoma
- Small adenocarcinoma
- Uncommon
- Infarcted bowel
- Pitfalls related to residual fluid
- Chronic Crohn’s disease
- Chronic radiation injury
Heterogeneous
Stratified attenuation
- Common
- Ischemia
- Infectious enterocolitis
- Crohn’s disease, ulcerative colitis
- Vasculitis, lupus, Henoch-Schönlein purpura
- Radiation
- Bowel edema related to cirrhosis or low-protein state
- Uncommon
- Infiltrating scirrhous carcinoma (usually stomach or rectum)
- Residual fluid and contrast material
- Submucosal fat deposition
- Pneumatosis
Mixed attenuation
- Common
- Large adenocarcinoma
- Gastrointestinal stromal tumor
- Mucinous adenocarcinoma
Length of Bowel Wall Thickening
Focal (<10 cm)
- Common
- Diverticulitis, appendicitis
- Adenocarcinoma
- Uncommon
- Lymphoma
- Tuberculosis
- Crohn’s disease
Segmental (10–30 cm)
- Common
- Lymphoma
- Crohn’s disease
- Infectious ileitis
- Radiation
- Submucosal hemorrhage
- Ischemia
- Uncommon
- Systemic lupus erythematosus
Diffuse
- Common
- Ulcerative colitis
- Infectious enterocolitis
- Edema from low protein and cirrhosis
- Systemic lupus erythematosus
- Uncommon
- Ischemia
Lymph Nodes
- The number, size, location, and attenuation of lymph nodes in the abdominal and pelvic cavities are important associated findings when examining patients with thickened bowel
- When present, especially in the sigmoid or descending colon, the main differential diagnosis is adenocarcinoma versus diverticulitis
- Pericolonic lymph nodes adjacent to the focal area of colonic thickening are more commonly seen in patients with colon cancer
- Pericolonic inflammatory changes are more commonly seen in diverticulitis
- Attenuation
- Low-attenuation lymph nodes with a rim of contrast enhancement or calcified lymph nodes should alert one to the possibility of tuberculosis, other mycobacterial infections, or histoplasmosis
- In a patient with AIDS, the presence of high-attenuation lymph nodes suggests the possibility of Kaposi's sarcoma.
- In this condition, the lymph nodes are hyperemic and will show enhancement during CT performed with IV contrast material
Signs of free air on supine film
- football sign
- lucency over the liver or at its inferior edge
- Rigler’s
- traingles or rhomboids of air b/w bowel loops
- air in lesser sac or morrison’s pouch, oulining of falciform
- in kids, air outlining umbilical ligaments or in scrotum
Retroperitoneal air
- results from trauma, inflammatory dz of retroperitoneal portions of GI tract, such as diverticulitis (asc colon, desc colon, rectum, parts of duodenum), BE, gas producing organisms, or from a renal process
- 3 spaces: perirenal, anterior pararenal and posterior pararenal
- anterior pararenal is most common and air outlines the lateral psoas margin and kidneys
- posterior pararenal comes from rectum or sigmoid and outlines the adrenals and part of the hemidiaphragm
- can cause pneumomediastinum and subcutaneous emphysema
- more fixed in location than peritoneal air (doesn’t change when pt moves) and it tends to be linear in shape
DDx psoas abscess
- TB spread from the spine(Pott’s dz) or kidney
- surgery
- hematogenous in immunocompromised pt
- IVDA
- colonic process such as diverticlutis
- Crohn’s
- appendicitis
- perforating neoplasm
- treatment is percutaneous drainage and antibiotics
Pneumatosis
- most common cause of portal venous gas is bowel infarction in adults or NEC in kids
- numerous other more benign causes such as penetrating ulcer, acute gastric or intestinal distension, IBD, iatrogenic from recent BE or endoscopy, and in babies from umbilical venous catheters
- pneumatosis intestinalis is a condition when air collects in the subserosa or submucosa of the bowel wall. It too has many causes, with ischemia being the most common
- other causes include surgery, NEC, Pseudomembranous colitis, IBD, infection, steroid users, Scleroderma, SLE and dermatomyositis, obstruction, trauma, BE/endoscopy and organ transplant, asthma CF or COPD
- can also be primary, which is a benign condition, and usually has rounded(submucosal) rather than linear(subserosal) gas collections
Intussusception
- invagination of one bowel loop into the other, with peristalsis pushing the proximal segment further into the distal one
- results in obstruction, ischemia or perforation
- children - 90% are idiopathic, probably related to lymphoid hyperplasia
- adults - overwhelming majority are related to an underlying lesion which serves as a lead point
- lead points include benign tumors such as lipoma, leiomyoma or adenoma, malignant tumors, meckel’s tic, prolapsed gastric mucosa, ectopic pancreatic rest, foreign body and feeding tubes
- can also be related to underlying diseases without lead points, including celiac, Whipple’s and scleroderma
- SBFT - coiled spring appearance
- US - target sign in TRV plane and pseudokidney in longitudinal
- CT - donut sign with fat b/w the 2 loops
Gallstone ileus
- 3 findings on KUB
- SBO
- Gallstone seen in RLQ
- Pneumobilia b/c of the biliary-enteric fistula
Lymphoma
- non-hodgkins lymphoma produces either multiple enlarged individual nodes or conglomerate nodal masses, which may encase vessels, displace organs or obstruct the ureters
- spleen is also commonly involved, with diffuse involvement leading to splenomegaly being more common than focal lesions
Carcinoid
- type of APUD tumor
- in the abdomen, they are most commonly found in the appendix, but most symptomatic ones are in the small bowel
- all are premalignant
- most occur in the ileum, especially the distal 2 feet, and carcinoid is the most likely cause for a submucosal mass in the ileum
- ileal carcinoid is the most aggressive, and the likelihood of mets increases with size
- >2cm have 90% chance of mets
- often multiple
- when it is a polyp, it is still potentially curable with resection
- with progression, there is growth into the mesentery and a desmoplastic response in the mesentery, with tethering, kinking and luminal narrowing
- narrowed segment with crowded folds leading to a curved segment with drawn out folds
- CT - mesenteric mass with curvilinear strands extending thru the mesentery toward surrounding SB loops, which have thickened walls
- carcinoid syndrome occurs when there are liver mets, in which case serotonin is directly secreted into the venous sytem without detox by the liver, and the pt gets bronchospasm, wheezing, diarrhea and flushing
- carcinoid mets to liver are hypervascular and enhance
- other hypervascular tumors include islet cell, melanoma, chorio, pheochromocytoma, breast and thyroid (CT CHIMP)
Abdominal Hernias
- Incisional
- Inguinal: direct (through Hesselbach’s triangle)/ indirect (along canal)
- Paraumbilical: through linea alba
- Spigelian: through linea semilunaris at lateral edge of rectus abdominis
- Lumbar hernias
- Paraduodenal hernias (= mesocolic hernias):
- #1 internal hernia (> paracecal; lesser sac; transmesenteric hernias)
- Right paraduodenal: behind hepatic flexure; fossa of Waldeyer; associated with cecal malrotation + Ladd’s bands; usually small contains portions of mesentery + vessels (middle colic artery + IMV)
- Left paraduodenal: fossa of Landzert near splenic flexure; often large; contain jejunal loops → mary become incarcerated → obstruction/ ischemia
- Obturator: SB → ↑ herniation/ strangulation; Howship-Romberg sign (compression of obturator nerve → pain along superomedial thigh)
Krukenberg tumors
- mets to the ovaries, usually from gastric or colon/appendix ca, breast ca, pelvic (CBS)
- in the setting of gastric or colon cancer, any cystic or solid lesion involving the ovaries should be considered mets until proven o/w.
gi.txt · Last modified: 2024/07/16 15:47 by 127.0.0.1